 | |
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
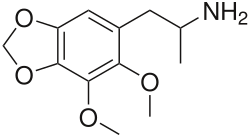
| Formula | C12H17NO4 |
| Molar mass | 239.271 g·mol−1 |
| 3D model (JSmol) |
|
| Melting point | 178 to 180 °C (352 to 356 °F) |
| |
 N N Y (what is this?) (verify) Y (what is this?) (verify) | |
DMMDA-2 is a bioactive phenethylamine discussed by Alexander Shulgin in his book PiHKAL (Phenethylamines i Have Known And Loved); however, he was not the first to synthesize it.[1] Shulgin comments in his book that a 50 milligram dose of DMMDA-2 produces similar effects to MDA.[1] DMMDA-2 can be synthesized from dillapiole.[1] DMMDA-2 is equivalent in potency to 5 mescaline units. DMMDA-2's isomer DMMDA is equivalent to 12 mescaline units.[2]
See also
- Substituted methylenedioxyphenethylamine
- Substituted methoxyphenethylamine
- Dimethoxymethylenedioxyamphetamine
- Methoxymethylenedioxyamphetamine
References
- ^ a b c "#59 DMMDA-2". PiHKAL: a chemical love story. Erowid. Retrieved 22 November 2011.
- ^ Clare BW (February 1990). "Structure-activity correlations for psychotomimetics. 1. Phenylalkylamines: electronic, volume, and hydrophobicity parameters" (PDF). Journal of Medicinal Chemistry. 33 (2): 687–702. doi:10.1021/jm00164a036. PMID 2299636. Retrieved 2025-01-07.
External links
- DMMDA-2 - Isomer Design
- DMMDA-2 - PiHKAL - Erowid
- DMMDA-2 - PiHKAL - Isomer Design